|
BIOPHYSICS OF THE PROVISIONAL MATRIX
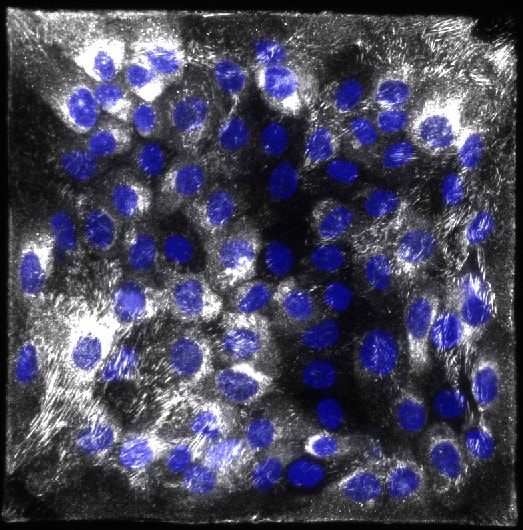
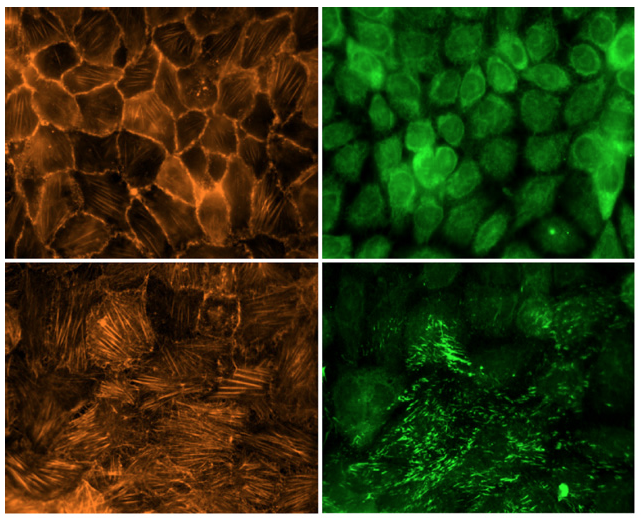
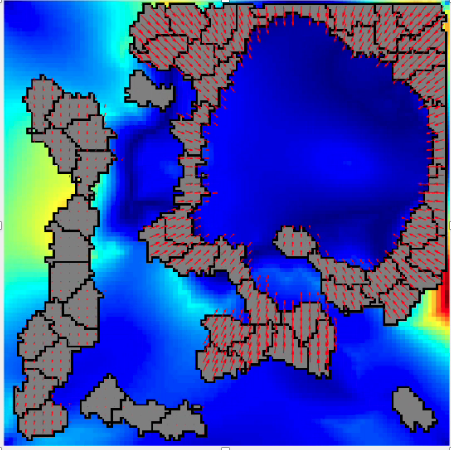
The provisional matrix is the term given to the temporary extracellular matrix that cells assemble during wound healing. Evidence indicates that in the setting of many diseases, including fibrotic diseases and cancer, cells assemble a similar matrix. This matrix is primarily composed of a protein known as fibronectin, which has unique mechanical properties that alter cellular response. We experimentally investigate both the effects of fibronectin fibrils on cellular mechanical responses and the mechanical properties of these fibronectin fibrils. |
GROWTH FACTOR SIGNALING IN THE PROVISIONAL MATRIX
Fibronectin fibrils in the provisional matrix are not simply structural elements; they also provide a platform to modulate growth factor signaling. Fibronectin contains a growth factor binding domain that can cluster growth factors in the provisional matrix. We are studying how this localization of soluble signals leads to altered cellular responses. We are particularly interested in how TGF-b1 interacts with fibronectin during fibrotic diseases and cancer. |
TISSUE-SPECIFIC CELL-MATRIX INTERACTIONS IN DISEASE
Interactions between cells and the surrounding matrix are specific to the tissue type. We are exploring how these interactions are distinct in different tissue settings. This includes development of an in vitro renal mimetic to study fibrosis in Chronic Kidney Disease, studies of the effects of hypoxia on the provisional matrix as is seen in solid tumors, and studies of the effects of the provisional matrix on neural progenitor cell differentiation. |
|
|
Our Story |
|
The Lemmon Lab at Virginia Commonwealth University specializes in cell-matrix mechanobiology. Our lab is interested in how cells assemble extracellular matrix to achieve the desired mechanical properties and signaling events necessary to promote cell attachment, organization, and function in tissues. We are also interested in dysregulation of this process in various disease states, including cancer, diabetes, and birth defects. We investigate these questions using an array of tools, including novel microfabricated structures that serve as a scaffold for ECM assembly and allow for measurement of cell-derived forces, proliferation, and migration in vitro; novel substrates with defined viscoelastic properties; and novel biological inhibitors of provisional matrix assembly and signaling.
|
|
connect with us |
|
AWARDS |
RECENT EVENTS |